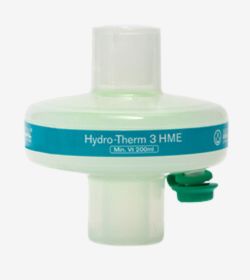
Breathing filters provide an effective barrier that prevent cross contamination between patients, breathing systems, respiratory and anaesthetic equipment, and the clinical environment. Their use is widely recognised as beneficial and is recommended by a number of Anaesthetic Associations1. They are widely used across the hospital, particularly in the operating theatre, critical care, lung function units, and respiratory care units.
Heat and Moisture Exchangers (HMEs) minimise loss from patient expired gases. When combined with a filter (Heat and Moisture Exchanging Filter, HMEF) this will also reduce the risk of cross contamination in the clinical environment.
Related Pages
- Filtration and Humidification
- Breathing filters
- HMEs and HMEFs
- Respiratory device filters
- Glossary
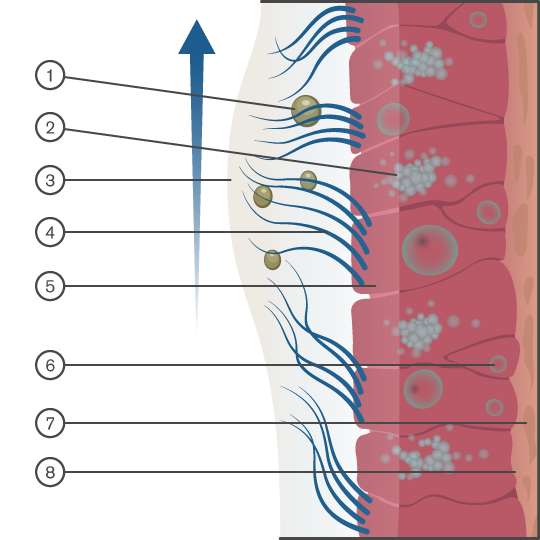
Movement of mucus to the pharynx
The normal function of the body’s upper airway is to filter, humidify and warm the air we breathe in. Air is warmed by passing over a vast network of capillaries in the nose. Mucous membranes line the upper airways and help to moisten the air and trap airborne particles (dust and microbes). Cilia move the mucus away from the lungs and towards the pharynx for removal.
The upper airways also help conserve heat and humidity, which would otherwise be lost during normal respiration. Gas leaving the lungs during expiration will be at body temperature (37°C) and have an Absolute Humidity (AH) of 44mg/l H2O and a Relative Humidity (RH) of 100%. As the patient breaths out, heat and moisture is retained by the upper airways and then transferred to the inspired gas, which is normally cooler and drier depending upon the ambient conditions. The large surface area of the upper airways makes it particularly efficient. This helps minimise any potential side effects associated with breathing cold dry gases over a prolonged period.
This natural physiological protection is bypassed when an artificial airway device is inserted, for example a tracheal tube, a supraglottic airway or tracheostomy tube. This means the gas source has a direct route to the delicate and sensitive lungs, which can result in an increased risk of infection and potential microbial cross contamination.
It could also result in a cooling and drying out of the airways as a result of breathing cold dry medical gases over a prolonged period of time. Room air would normally be approximately 23°C with an RH of 60% and an AH of 21mg/l. Medical gases would normally be 10-15°C with a RH of 0-2 % and an AH of 0.5 mg/l, which would further increase the risk. This can lead to damaged cilia, thicker mucous, mucous crusts leading to an increased risk of tube occlusion, increased risk of infection, atelectasis and increased cost due to prolonged hospital stay.
Diagram Key: 1. Particulate. 2. Mucous cell 3. Mucus layer 4. Cilia 5. Ciliated columnar epithelial call. 6. Stem cell 7. Lamina propria 8. Basement membrane
Clinical environment
Infection
Protecting the patient
Critically ill patients with reduced immunity are commonly at an increased risk of infections. These nosocomial infections result in increased morbidity and potential mortality as well as having a significant impact on the cost of treating the patient due to increased hospital stay. The strategic use of an efficient, properly validated breathing filter provides an effective barrier between patient, breathing systems and respiratory equipment, which reduces the risk of cross contamination.
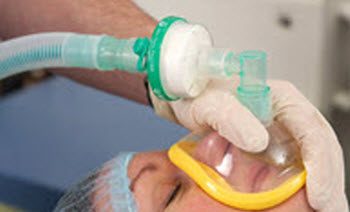
Cross contamination
Protecting the breathing system
Those patients who require an artificial airway have their natural physiological protection by-passed. This will increase the risk of infection and cross contamination between patients and healthcare equipment. The cross contamination of patients via an anaesthetic system has been reported and documented(2). Areas of concern regarding infection include Hepatitis C, Mycobacterium tuberculosis, blood in sputum, SARS and similar coronaviruses.
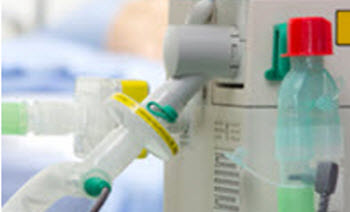
Protection of respiratory equipment and clinical enviroment
Protecting the equipment
The use of appropriate and effective breathing filters can provide protection to delicate and expensive equipment, helping to preserve functionality, reduce running costs and reduce potential cross contamination.
Proven efficiency
Increasingly the only way that a clinician can determine the efficiency and effectiveness of the performance of a breathing filter, HME and HMEF is via standard test protocols and results. Ensuring the data is clinically relevant, up-to-date and reflective of the product that the customer is using is vital.
Our range of breathing filters and HMEFs have been independently tested and proven to be highly efficient in preventing the passage of bacteria and viruses. These tests provide clinically relevant information to allow evidence-based decisions to be made on the most appropriate product to meet your clinical requirements.
Microbiological testing
All our filters are tested at specialist microbiology laboratory facilities against clinically relevant bacterial and viral challenges. This is normally performed at an independent test facility that develops specific protocols to simulate the types of challenges that a filter may see in the clinical setting.
A challenge particle is chosen to simulate the size of the commonly occurring bacteria and viruses. Each individual Intersurgical filter and HMEF is tested and their performance verified in this way.
Clinically relevant testing is carried out on all products using Bacillus subtilis (1.0µm x 0.7µm) and Ø174 bacteriophage (0.027µm).
Watch our microbial challenges video below to see how our breathing filters and HMEFs protect against different particle sizes.
Potential infectious viruses [Particle sizes μ microns]
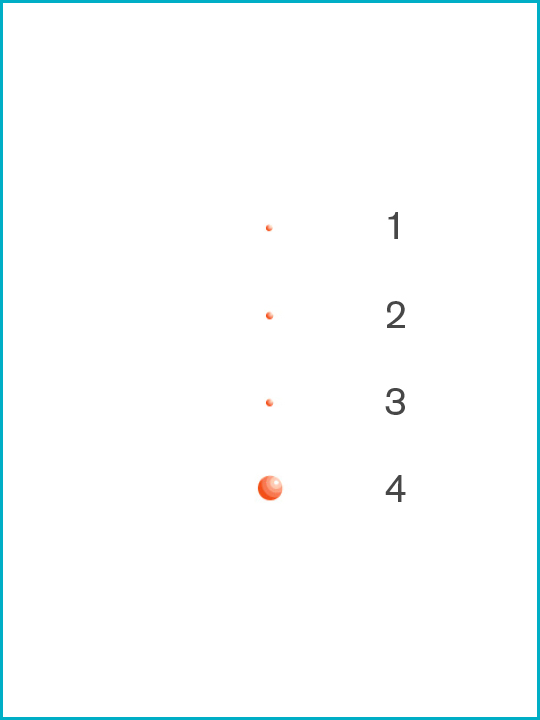
1. Coliphage T1 [0.017μ]
2. MS-2 coliphage [0.02μ]
3. Hepatitis C [0.03μ]
4. Adenovirus [0.07μ]
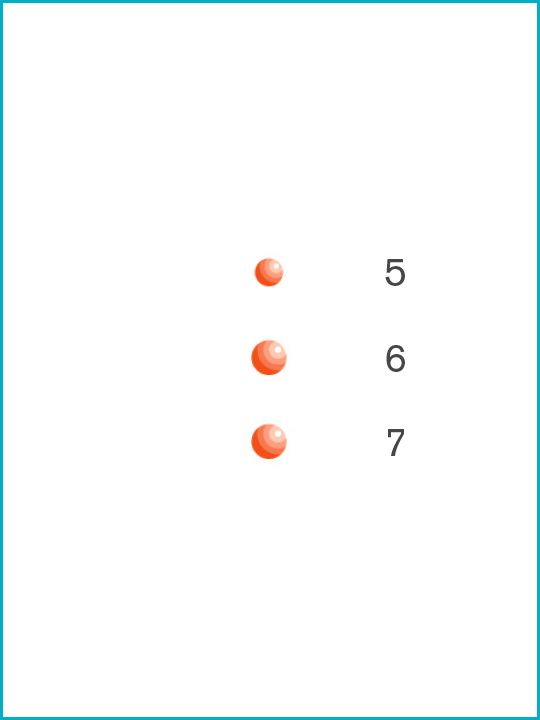
5. HIV [0.11μ]
6. Orthomyxovirus [0.1μ]
7. Cytomegalovirus (CMV) [0.1μ]
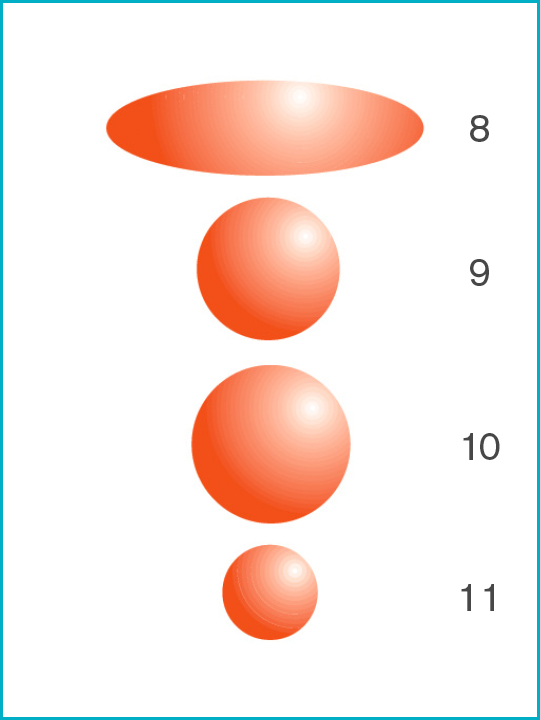
8. Mycobacterium tuberculosis [0.3μ x 1.0μ smallest size]
9. Serratia marcescens [0.45μ]
10. Pseudomonas aeruginosa [0.5μ]
11. Brevundimonas diminuta [0.3μ]
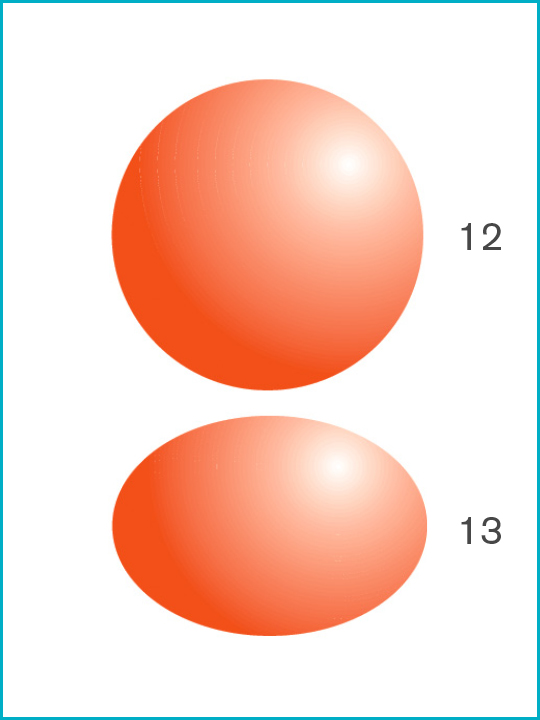
12. Staphylococcus aureus [1.0μ]
13. Bacillus subtilis [1.0μ x 0.7μ]
The level of breakthrough of the challenge determines the efficiency. This efficiency is reported as a percentage based upon this break-through when compared to the challenge.
| Number of organisms challenging the filter | Number of organisms passing through the filter | Efficiency of the filter |
| 100,000 | 1000 | 99% |
| 100 | 99.9% | |
| 10 | 99.99% | |
| 1 | 99.999% |
The challenge presented in the viral test protocol (φ174 bacteriophage, 0.027 μm) will be at least as severe as that posed by the Coronavirus (COVID-19). Performance statements are available upon request for individual products.
The COVID-19 outbreak has affected the majority of countries and sadly lead to thousands of deaths globally.
The range of Intersurgical breathing filters and HMEF's have been independently tested and proven to be highly efficient at preventing the passage of bacteria and viruses in the healthcare environment.

All products that have a filter have been tested in accordance with and comply with the requirements of ISO 23328-2003.
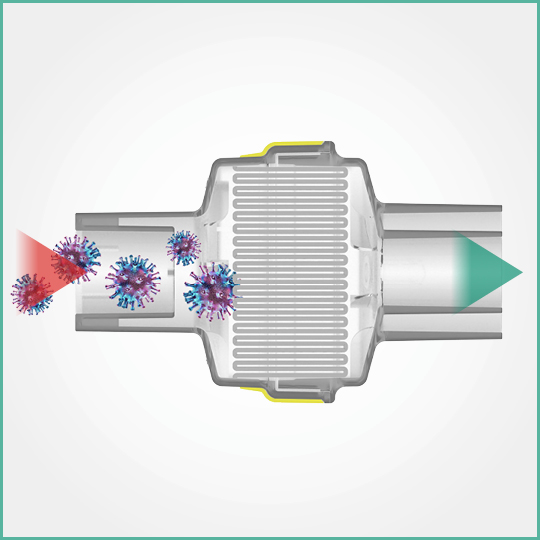
On the basis of the size and shape of the COVID-19 corona virus (0.05-0.1 micron) and the more severe viral test data on file which uses a particle of 0.027 micron challenge, it can be confirmed that the Intersurgical range will provide at least the same level of protection against COVID-19 as reported in the independent microbiological tests. Copies available upon request.
Colour coded for ease of selection
Our range of breathing filters, Heat and Moisture Exchangers (HMEs) and Heat and Moisture Exchanging Filters (HMEFs) are colour coded for ease of identification and to ensure the correct filter/HME is used, minimising any potential risk to the patient.
Reference 1: Association of Anaesthetists of Great Britain and Ireland 1996. Danish Society of Anaesthetists 1998. French Society of Anaesthetists 1998
Reference 2: Chant K, Kociuba K, Munro R, et al. Investigation of possible patient-to-patient transmission of hepatitis C in a hospital. NSW Public Health Bull 1994; 5:47-51.